(WJW) – More than 2,000 bottles of liquid Benadryl are being recalled due to a risk of child poisoning, according to a recall notice published by the U.S. Consumer Product Safety Commission.
The recall alert says Benadryl contains diphenhydramine, an antihistamine, and “must be in child-resistant packaging as required by the Poison Prevention Packaging Act.” The packaging, however, does not meet these standards, posing a possible risk of poisoning, the notice says.
The recalled bottles were sold on Amazon.com from July 2023 through October 2024, according to the CPSC.
Despite the packaging issue, there have been no reports of poisonings.
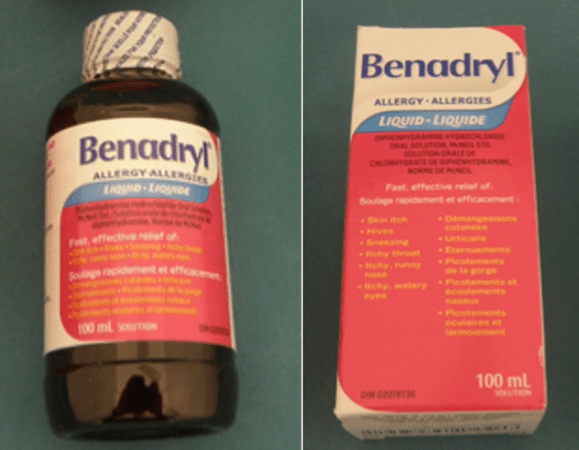
The recall concerns 100 mL bottles of Benadryl Liquid Elixir. The products can be identified by the following specifications:
- Packaged in a paper box decorated with pink and white with the word “Benadryl” written in blue text
- Packaged in a round dark plastic bottle with pink and white label on the front with the word “Benadryl” in blue text
- Contains a white label on the bottom with the following code written in black text: “X003VRIGUL.”
Consumers are being urged to keep the recalled Benadryl “out of the sight and reach of children,” according to the notice.
Arsell, the company listed as the product’s importer, can be contacted for a full refund. Customers will be asked to submit their Amazon order number and a photo demonstrating disposal of the recalled Benadryl to recall@arsellsupport.com.
“Only the bottle is being recalled, not the medicine itself, but both should be disposed of,” reads the notice. “All known purchasers are being contacted directly.”